CNHHE
CMP News
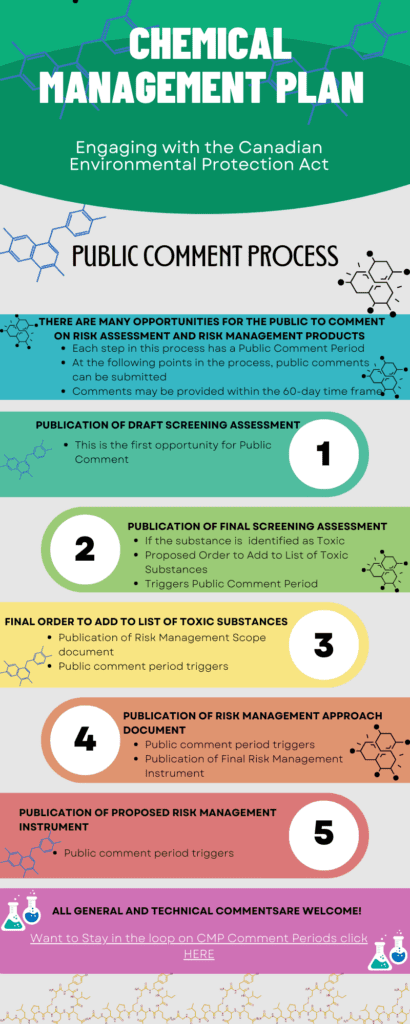
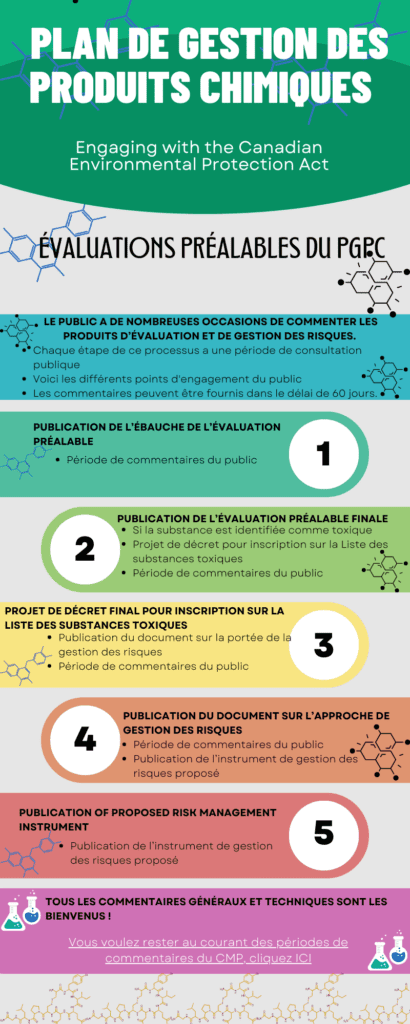
Once a chemical is found to pose a risk, scientists and policy people with the Chemicals Management Plan design a strategy to manage the chemical and the risk it poses. They might decide that it is important to phase out the use or production of the substance because it is too dangerous. They might propose regulations to limit or make the use of a chemical safer.
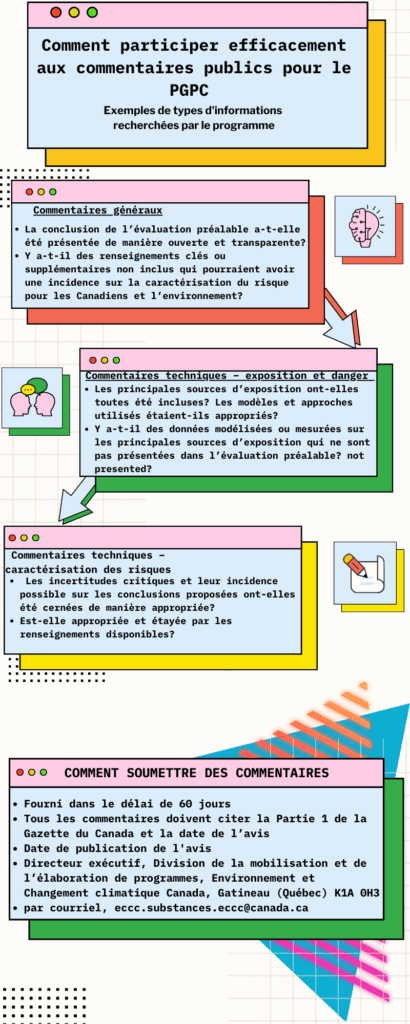
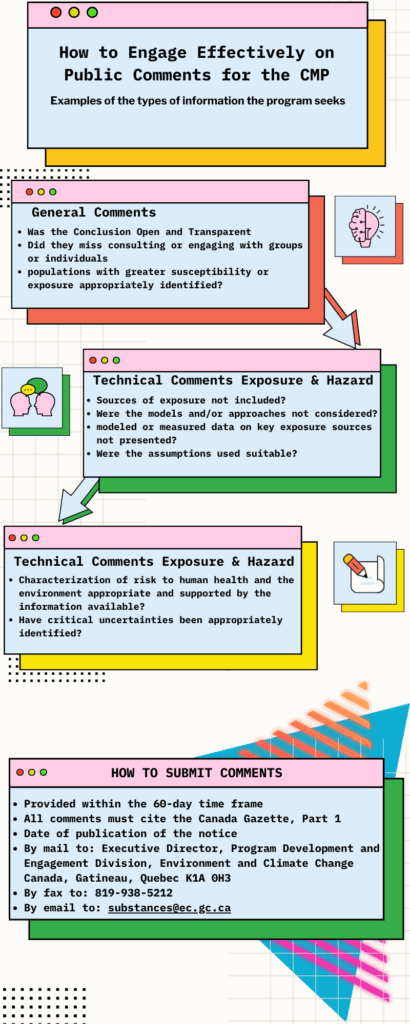
At every step along the way there are Public Comment Periods open to the people of Canada to comment on a draft document about a chemical. These comments, and the CMP Science Committee and Stakeholder Advisory Council help the government make decisions about how to manage a chemical or group of chemicals for the safety of Canadians and our environment.

Toxics Caucus
The Toxics Caucus has a mandate to link local and global activities, public and private sectors, and nonprofit actors in their work. In accordance with the Caucus’s mandate and Memorandum of Understanding with the New Brunswick Lung Association and Health Canada, the goal is to support Canadian Civil Society Organizations and disproportionately impacted populations to engage in the Chemicals Management Plan (CMP) and environmental health initiatives.
The Caucus is an apolitical advocate based on a democratic majority among members, for actions that improve environmental and human health. The Caucus represents its members, as a union of interested parties, much as trade associations act in relation to the private sector.
Latest from the CMP
Newsletter 2023-03-08
Updates on webinars and infographics
CMP Infographics
We have made some infographics to show how you can be involved in the process and how the CMP process works
Nitrilotriacetic Acid Trisodium Salt
The Final Screening Assessment for Nitrilotriacetic Acid Trisodium Salt was published. A notice of intent to amend the Domestic Substances List to apply the Significant New Activity (SNAc) provisions of the Canadian Environmental Protection Act, 1999 to Nitrilotriacetic Acid Trisodium Salt was also published.
Sodium ortho-phenylphenate
The Final Screening Assessment for [1,1’-Biphenyl]-2-ol, sodium salt (Sodium ortho-phenylphenate; SOPP) was published. A notice of intent to amend the Domestic Substances List to apply the Significant New Activity (SNAc) provisions of the Canadian Environmental Protection Act, 1999 to the Sodium ortho-phenylphenate was also published.
Nitrilotriacetic Acid Trisodium Salt
The Final Screening Assessment for Nitrilotriacetic Acid Trisodium Salt was published. A notice of intent to amend the Domestic Substances List to apply the Significant New Activity (SNAc) provisions of the Canadian Environmental Protection Act, 1999 to Nitrilotriacetic Acid Trisodium Salt was also published.
Supply Chain Transparency
Notice of intent on the labelling of toxic substances in products, including toxic flame retardants was published on October 29, 2022.
Poly(alkoxylates/ethers) Group
The Final Screening Assessment for the Poly(alkoxylates/ethers) Group was published.
Thiocarbamates Group
The proposed order adding TMTD to Schedule 1 of the Canadian Environmental Protection Act, 1999 was published for a 60-day public comment period ending on December 7, 2022.
Dichloromethane
The performance measurement evaluation for Dichloromethane (DCM) / Methylene Chloride was published.
Aldehydes Group
The Draft Screening Assessment for the Aldehydes Group was published for a 60-day public comment period ending on December 7, 2022.
Protein Derivatives and Yeast Extract Group
The Final Screening Assessment for Protein Derivatives and Yeast Extract Group was published.
Get Your Letter on the NatureBUS
Be a voice for Nature! Get your message on the NatureBus to NatureCOP in Montreal. Tell our
leaders to protect global biodiversity.
Nature Canada’s NatureBus tour is heading to the NatureCOP conference in Montreal. This
In December, officials from 195 countries and the European Union will gather to discuss global
progress on nature protection and come up with a plan to halt and reverse nature loss by 2030.
The NatureBus will be making stops all over the country to collect your messages of support for
a plan to restore nature. On December 6, they’ll deliver the messages to the Prime Minister at
the NatureCOP conference in Montreal.
Come meet the NatureBus today and deliver your letter in person! Send your
message of hope for Nature to NatureCOP.
Your message of hope will be featured in our NatureCOP installation: “Letters of Hope –
Canadians’ support and wishes for a nature-positive world” to showcase Canadians’ support
and urge Canadian leadership to ensure successful NatureCOP outcomes.
If you can’t make it to the bus, send a digital message to make sure your voice is heard by world
leaders at NatureCOP!
You can DOWNLOAD all the resources below as well!
Template Letter to Prime Minister
Hashtags options
#NextStopNatureCOP #NatureBus #dontCOPout #NatureCOP #COP15 #ForNature #30×30 #NatureBasedSolutions
Information from Health Canada Regarding the Consumer Prod ucts Containing Lead Regulations
Are the consumer products you manufacture, import, advertise or sell in Canada subject to the requirements under the Consumer Products Containing Lead Regulations?
The Regulations were amended in 2018 to expand their scope to include:
- products intended for use in learning or play (toys) by children under 14 years of age;
- clothing or clothing accessories intended for use by a child under 14 years of age; and
- products whose primary purpose is to facilitate the relaxation, sleep, hygiene, carrying or transportation of a child under 4 years of age.
Health Canada has published Information for Regulated Parties Regarding the Consumer Products Containing Lead Regulations to provide more details on:
- examples of products in scope of the Regulations,
- exceptions, and
- test methods.
You can consult the document at the following links:
https://www.canada.ca/en/health-canada/services/consumer-product-safety/legislation-guidelines/guidelines-policies/consumer-products-lead-regulations.html
Silver and its Compounds
The Final Screening Assessment for Silver and its Compounds was published.
The Final Screening Assessment for Silver and its Compounds was published.
Share and view ideas: Consultation on proposed regulations for polycyclic aromatic hydrocarbons (PAHs) in sealant products
The consultation on proposed regulations for polycyclic aromatic hydrocarbons (PAHs) in sealant products was published for a public consultation period, ending on October 22, 2022.
https://canada.ca/en/environment-climate-change/corporate/transparency/consultations/polycyclic-aromatic-hydrocarbons.html
Federal Environmental Quality Guidelines (FEQGs)
Summaries of the public comments received on the Federal Environmental Quality Guidelines for Siloxane-D4, Selenium, and Aluminum were published.
https://www.canada.ca/en/health-canada/services/chemical-substances/fact-sheets/federal-environmental-quality-guidelines.html
Webinar: Applying Economic Insight to Environmental Health Risk Management March 30th, 2022 1pm AST 12pm EST
The webinar is being presented by Wambui Kipusi, Healthy Environments and Consumer Safety Branch, Health Canada. She previously did economic analysis linked to climate change, air pollution and toxic chemicals at Environment and Climate Change Canada. She did her graduate studies in economics, business and policy at Dalhousie University.
The webinar will examine how Governments often take policy actions intended to reduce human health risks from environmental contaminants deemed to adversely affect the wellbeing of Canadians. Assessing the impacts of these actions, and determining whether they are beneficial, can be complex and time-consuming. In order to inform decision-making and subsequent policy choices, governments draw on expertise from many disciplines including science, engineering and economics. This presentation will provide:
- An overview of how economics informs environmental health risk management actions
- Application examples
Fluorescent brightener 367
| The Final Screening Assessment for Benzoxazole, 2,2′-(1,4-naphthalenediyl)bis- was published. | This substance was identified for action under the Chemicals Management Plan (CMP). The screening assessment focused on benzoxazole, 2,2′-(1,4-naphthalenediyl)bis-, also referred to as fluorescent brightener 367, which was assessed under the third phase of the Chemicals Management Plan (CMP). Fluorescent brightener 367 does not occur naturally in the environment. According to information gathered by the Government, it was not manufactured or imported into Canada above the reporting thresholds. Fluorescent brightener 367 may be found in cosmetics, such as certain nail polishes. |
1-Nitropropane
| The Final Screening Assessment for 1-Nitropropane | This substance was identified for action under the Chemicals Management Plan (CMP). |
The Draft Screening Assessment for Select Hydrocarbon-based Substances was published for a 60-day public comment period ending on March 9, 2022.
The screening assessment summarized here focuses on 8 hydrocarbon-based substances, assessed under the Chemicals Management Plan (CMP). The substances addressed are petroleum resins, hydrocarbon resin, polymerized C5-12 distillates, oxidized hydrocarbon waxes with ethanolamine, oxidized hydrocarbon waxes with 2-(methylamino)ethanol, alkylated naphthalene sulfonate sodium salt polymers with formaldehyde, heavy oxo ends, and sulfurized petroleum. To learn more about The Draft Screening Assessment for Select Hydrocarbon-based Substances was published for a 60-day public comment period ending on March 9, 2022, click here.
To inquire about honorarium to support the CMP mandate, please contact Andrew Holloway M.Sc at the New Brunswick Lung Association via email at andrew.holloway@nb.lung.ca
Updated Pre-Budget Consultation
Updated pre-budget consultation
Feb 25, 2022
RESILIENT has submitted a pre-budget consultation brief to the Federal Department of Finance with our recommendations for Canada to emerge from the COVID-19 pandemic healthier and more resilient.
“Human Health, the economy, and our environment are inextricably linked”
This process echoes submissions to the Federal Finance Committee made in August 2021. Our recommendations continue to centre on:
- Taking strong action on climate change by diverting fossil fuel subsidies into the green economy and a just transition for the fossil fuel workforce, and accelerating the adoption of low carbon commuting
- Preventing climate change mitigation from increasing air pollution by including radon mitigation in energy efficiency rebates and mandating a reduction in wood smoke pollution
- Launching a national pharmacare system
- Modernizing the Canadian Environmental Protection Act (CEPA) in line with current science
- Implementing a recovery basic income
We are encouraged that Bill C-28 (and Act to Modernize CEPA) that was introduced in April 2021 we re-instated in the Senate as Bill S-5 in the current session of Parliament. We urge that modernization to CEPA go forward without delay.
To read our full written submission for the pre-budget consultations click here
To read 2021 written submission for the pre-budget consultations click here
Sixth Report on Human Biomonitoring of Environmental Chemicals in Canada
Sixth Report on Human Biomonitoring of Environmental Chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 6 (2018-2019), along with 8 biomonitoring fact sheets for a selection of chemicals.
A technical briefing was held this morning presenting highlights from the report and fact sheets. A PDF copy of the presentation is attached.
The Canadian Health Measures Survey (CHMS) is a comprehensive direct health measures survey conducted by Statistics Canada, in partnership with Health Canada and the Public Health Agency of Canada. The CHMS includes a biomonitoring component, funded by Health Canada, which measures the levels of environmental chemicals in individual samples of blood and urine. Health Canada’s National Biomonitoring Program reports are key deliverables under the Chemicals Management Plan.
The Sixth Report contains summary data for 79 environmental chemicals measured in cycle 6 of the CHMS. It features data for the same set of chemicals presented in the previous Fifth Report, except for volatile organic compounds (VOCs), which were excluded for cycle 6. The report does not address any health outcomes, provide analyses or conclusions, or recommend policy direction. It is strictly a technical document that outlines objectives, describes the survey design and methods, and provides descriptive summaries of measured environmental chemicals, along with approximately 130 data tables.
New this year is the publication of biomonitoring fact sheets that highlight changes in chemical exposures over time, distributions across age groups, differences between males and females, and comparisons between the Canadian population and other populations in Canada and the United States. Eight fact sheets have been released this year on arsenic, cadmium, lead, mercury, PFAS, DEHP, bisphenol A (BPA) and parabens.
Data files are also available through Statistics Canada’s Research Data Centers (RDCs).
Methylstyrenated phenol
| Draft Screening Assessment Risk Management | The draft screening assessment for methylstyrenated phenol has been published. It is proposed to conclude that the substance may be harmful to the environment and meets the criteria under paragraph 64(a) of the Canadian Environmental Protection Act, 1999 (CEPA). A risk management scope has been published concurrently to initiate discussions with stakeholders on the risk management options being considered. |
Flame Retardants Group
| Draft Screening Assessment Risk Management Scope | The draft screening assessment for the Flame Retardants group has been published. It is proposed to conclude that IPPP may be harmful to the environment and to human health and meets the criteria under paragraphs 64(a) and (c) of CEPA. It is also proposed to conclude that TPHP, BPDP, BDMEPPP and IDDP may be harmful to the environment and meet the criteria under paragraph 64(a) of CEPA, while TEP may be harmful to human health and meets the criteria under paragraph 64(c) of CEPA. A risk management scope has been published concurrently to initiate discussions with stakeholders on the risk management options being considered. It is also proposed to conclude that the remaining six substances do not meet any of the criteria set out in section 64 of CEPA. |
Approach for a Subset of Organic and Inorganic Substances Prioritized Under the Chemicals Management Plan
| Science Approach Document Webpage | This approach addresses 9 organic and inorganic substances that were identified as priorities for assessment as they met the categorization criteria under subsection 73(1) of the Canadian Environmental Protection Act, 1999 (CEPA) or were considered a priority based on other human health concerns. It is proposed that these substances not undergo further assessment at this time. |
Priority B (medium hazard) micro-organisms on the Domestic Substances List
Priority B (medium hazard) micro-organism strains on the Domestic Substances List (DSL) were given a medium priority for assessment because while they are not specifically identified as pathogens, the Government of Canada identified evidence in the scientific literature that indicates they may have the potential to cause harm to human health or the environment. More information about the prioritization of micro-organisms on the Domestic Substances List is available. Information on all micro-organism assessments is also available. More information here
Canada’s Chemicals Management Plan (CMP)

Canada’s Chemicals Management Plan (CMP) program is sharing the following Publications Plan to inform stakeholders of publications from October to December 2021. Please note that the targeted publication dates are subject to change. The program anticipates sharing the Publications Plan for January to March 2022 by winter 2021.
Environment and Climate Change Canada and Health Canada recognize the impacts faced by Canadians in light of current events and understand the importance of stakeholder engagement. If you anticipate any challenges with providing your comments within the 60-day public comment period, if applicable, please inform the program at the earliest opportunity.
| Publications targeted for October to December |
| Acids & Bases DSAR a |
| Approach for a Subset of Organic and Inorganic Substances Prioritized under the Chemicals Management Plan b |
| Flame Retardants DSAR a |
| Phenol, methylstyrenated DSAR a |
a Draft Screening Assessment Reports (DSAR) presenting information on health and ecological considerations and, where criteria under s. 64 are proposed to be met, Risk Management Scope documents.
b Science-based policy approach document.
Note: Group names are not final and may change
To inquire about honorarium to support the CMP mandate, please contact Andrew Holloway M.Sc at the New Brunswick Lung Association via email at andrew.holloway@nb.lung.ca
Webinar Registration – Sep 29, 2021 02:00 PM
Topic: Per- and Polyfluorinated Alkyl Substances (PFAS): A Review “From PFOS to GenX”DescriptionCNHHE is pleased to present Terry Obal, PhD, CChem, Chief Science Advisor, Bureau Veritas Laboratories to discuss the state of the science around PFAS chemicals.
Perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA) and related per- and polyfluorinated alkyl substances (PFAS) are recognized by environmental practitioners and regulatory bodies around the world as compounds of environmental concern. These persistent chemicals are found widely in the environment. The ubiquity of these compounds in a wide range of environmental and biological matrices, compounded with their chemical properties and studies suggesting human toxicity have resulted in increased public concern.
In April 2021, the Government of Canada published a notice of intent in the Canada Gazette considering activities that would address the broad class of PFAS.
This presentation will provide a brief overview of the science around PFAS including:
• PFAS as contaminants of emerging environmental concern
• Exposure and toxicity
• Sampling and analysis considerations – Soil, water, tissue and air
• Treatment and remediation – What are our options?
• The future of PFAS in the environmental marketplace
Lotus corniculatus, extract
The final screening assessment for Lotus corniculatus, extract has been published. It is concluded that the substance does not meet any of the criteria set out in section 64 of CEPA. The Final Screening Assessment is HERE & The Canada Gazette Notice is HERE
Comments or information submitted with respect to the above publication must cite the Canada Gazette, Part I, along with the date of publication of the notice, and can be provided to the Minister of the Environment until October 13, 2021, using one of the following methods:
- Online through Environment and Climate Change Canada’s Single Window,
- By email to substances@ec.gc.ca, with the name of the publication in the subject line. If you wish to comment on multiple publications, please submit your comments in separate emails; or
- By mail to the Executive Director, Program Development and Engagement Division, Environment and Climate Change Canada, 351 St-Joseph Blvd., 6th floor, Gatineau (QC) K1A 0H3.
Caprolactam
| The draft screening assessment for caprolactam has been published. It is proposed to conclude that the substance does not meet any of the criteria set out in section 64 of the Canadian Environmental Protection Act, 1999 (CEPA). The Draft Screening Assessment is found HERE and the Canada Gazette Notice can be located HERE |
Comments or information submitted with respect to the above publication must cite the Canada Gazette, Part I, along with the date of publication of the notice, and can be provided to the Minister of the Environment until October 13, 2021, using one of the following methods:
- Online through Environment and Climate Change Canada’s Single Window,
- By email to substances@ec.gc.ca, with the name of the publication in the subject line. If you wish to comment on multiple publications, please submit your comments in separate emails; or
- By mail to the Executive Director, Program Development and Engagement Division, Environment and Climate Change Canada, 351 St-Joseph Blvd., 6th floor, Gatineau (QC) K1A 0H3.
Long-chain Perfluorocarboxylic Acids
A Health science summary: Long-chain perfluorocarboxylic acids (LC-PFCAs) their salts and related compounds was published. To Read the Summary Click Here.
Plastic Pollution
A summary of feedback received on the discussion paper: A proposed integrated management approach to plastic products was published. Feedback can be found here.
Anthraquinones Group
The Final Screening Assessment for the Anthraquinones Group was published. The Proposed Risk Management Approach for Solvent Violet 13 was also published for a 60-day public comment period ending on September 15, 2021. Available here.
Mitotane
An order amending the Domestic Substances List to apply the Significant New Activity (SNAc) provisions of the Canadian Environmental Protection Act, 1999 to Mitotane was published. Available here.
Corn, steep liquor
The Final Screening Assessment for Corn, steep liquor was published. Available here.
Lead
The proposed Regulations for Prohibiting the Manufacture and Import of Wheel Weights Containing Lead in Canada were published for a 70 public comment period ending September 11, 2021. Available here.
Formaldehyde
The final Formaldehyde Emissions from Composite Wood Products Regulations and the final Directive Concerning Testing for Formaldehyde Emissions were published. Available here.
Triazines and Triazole Group
The Final Screening Assessment for Triazines and Triazole Group was published. Available here.
Zinc
The Additional Risk Characterization Document in Support of the Draft Screening Assessment for Zinc and its Compounds: Pulp and paper sector exposure and risk characterization was published. Available here.
Fact Sheets
Two new fact sheets were published: 1. Use of margins of exposure and risk quotients in risk assessment, and 2. Canadian exposure factors used in human health risk assessments. Available here.
Coal Tars
The Final Screening Assessment for Coal Tars and their Distillates was published. The Risk Management Approach for Coal Tars and their Distillates and the proposed order adding coal tars to Schedule 1 of the Canadian Environmental Protection Act, 1999 were also published for a 60-day public comment period ending on August 25, 2021. Available here.
DEHP
The performace measurement evaluation for DEHP was published. Available here.
Priority A (higher hazard) micro-organism
A notice of intent to amend the Domestic Substances List to vary the requirements under the Significant New Activity (SNAc) provisions of the Canadian Environmental Protection Act, 1999 for Pseufomonas fluorescens ATCC 13525 was published for a 60-day public comment period ending on August 18, 2021. A notice of intent to amend the Domestic Substances List to rescind the requirements under the SNAc provisions for Pseudomonas aeruginosa strains ATCC 31480, 700370 and 700371 was also published for a 60-day public comment period ending on August 18, 2021. Available here.
Siloxane-D4, Selenium, and Aluminum
Federal environmental quality guidelines for Siloxane-D4, Selenium, and Aluminum were published. Available here.
Chlorocresol
The Final Screening Assessment for Chlorocresol was published. The Proposed Risk Management Approach for Chlorocresol was also published for a 60-day public comment period ending on July 21, 2021. Available here.
Talc
The proposed order adding Talc to Schedule 1 of the Canadian Environmental Protection Act, 1999 was published for a 60-day public comment period ending on July 21, 2021. Available here.
Plastic manufactured items
A final order adding plastic manufactured items to Schedule 1 of CEPA 1999 was published. Available here.
Selenium and its compounds
A final order adding selenium and its compounds to Schedule 1 of CEPA 1999 was published. Available here.
Monocyclic and Bicyclic Sesquiterpenes Group
The Draft Screening Assessment for the Monocyclic and Bicyclic Sesquiterpenes Group and the Risk Management Scope for Certain Terpenes and Terpenoids within the Monocyclic and Bicyclic Sesquiterpenes Group were published for a 60-day public comment period ending on July 7, 2021. Available Here.
Chemicals Management Plan Science Committee Reports
The meeting record for the February 2020 Chemicals Management Plan Science Committee meeting was published. A committee report on considerations for identifying potential risks from exposure to chemicals in the workplace was also published. Available Here.
